Plasma fractionation industry producing so called IVIG products (Intra Venous Immuno globulin type G) are also facing serious challenges with the presence of anti A and anti B antibodies in their products (hemolysis accidents are more and more frequent and regulation will probably shortly impose complete removal of isoagglutinins). GlycoBAR offers several convenient and easy to use solutions to purify these IVIG from blood group antibodies.

The first one is by using a traditional chromatographic column with a resin grafted with GlycoBAR ligands. By adding a very simple and well known chromatographic purification step in their IVIG production process, IVIG suppliers can now eliminate completely isoagglutinins from their products and therefore provide a such safer solution to hospital for treating immune deficient patients. GlycoBAR has a partnership with a leading chromatographic resin supplier which is taking care of the grafting process and offer a turn-key solution to IVIG suppliers
Another option to eliminate isoagglutinins from IVIG is using a GlycoBAR ready to use and disposable solution. We can indeed propose our antigen sugars grafted on a cellulosic support that can be mixed with IVIG. A simple filtration process after a short incubation time would allow to obtain an IVIG product fully purified from blood group antibodies. GlycoBAR fermentation process needed to obtain the blood group antigens is so inexpensive that a such a simple and disposable solution is economically perfectly acceptable.
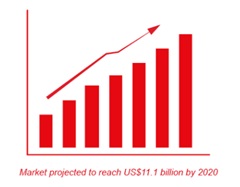
The worldwide IVIG market is planned to be around 20 tons in 2020.