March 2022:
GlycoBAR has identified new premises located in Domène, close to Grenoble France and will therefore move to it when retrofit will be completed and new equipment will be installed, probably at the end of 2023.

May 2021:
After a difficult Covid period where GlycoBAR activity has been severely slowed down, a new bright horizon is opening up: thanks to a grant from « France Relance » incentive program from the French gouvernement, GlycoBAR will shortly launch a scale-up program

May 2019:
Once again, GlycoBAR is participating to the semi annuel Plasma Product Biotechnology Meeting, which is held this year in Sicilia. This is a good time to meet with all the plasma business players.

January 2019:
GlycoBAR manufacturing organization successfully passed the ISO9001 certification audit.


August 2018:
GlycoBAR has signed an an agreement with Thermofisher which is now buying GlycoBAR ligands. The ligands are grafted on a Thermofisher resin which is used by a German pharma firm to remove isoagglutinins from a proprietary plasma derived product which will shortly go into clinical tests.
GlycoBAR production facility has been audited by Thermofisher which gave a A+ rating to this audit.
May 2017:

GlycoBAR will be attending the tenth Plasma Product Biotechnology Meeting that will be held in Malta from May 15th, 2017 to May 18th, 2017, with a poster presentation called “Removal of Isoagglutinins at Large Scale using an Innovative & Patented Oligosaccharides Production Process”
The poster can be downloaded from this link:
Novembre 2016:
GlycoBAR has received a special award from the European Commission called “Seal of excellence”. This award is recognizing the high potential of GlycoBAR technology in its targeted market.


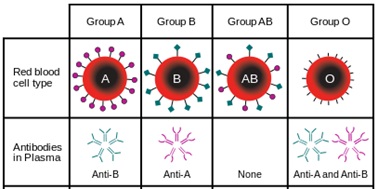
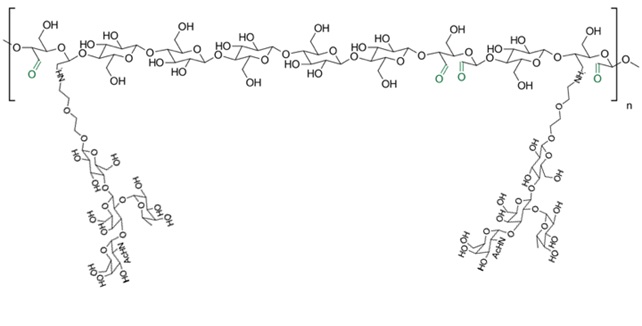
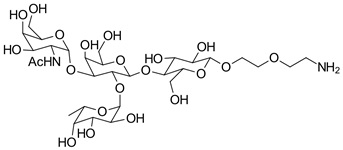
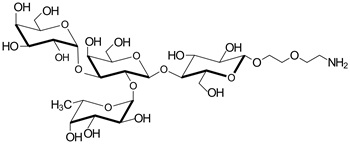
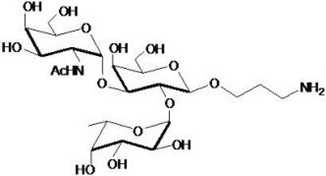
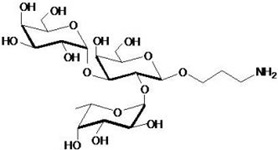
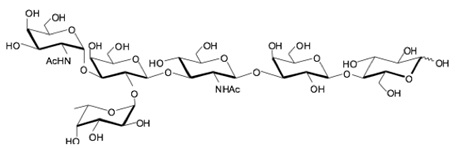
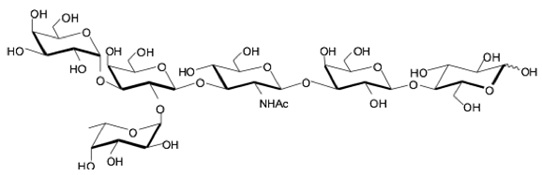
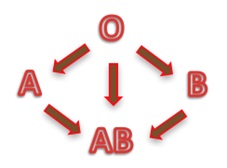
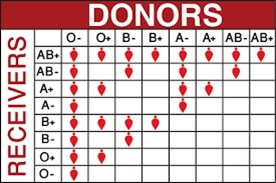
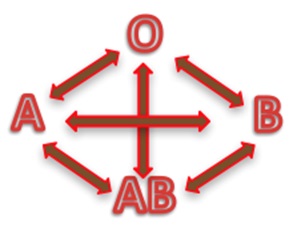
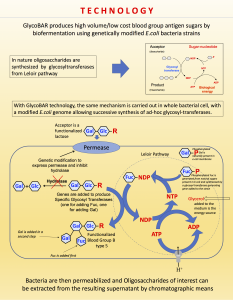
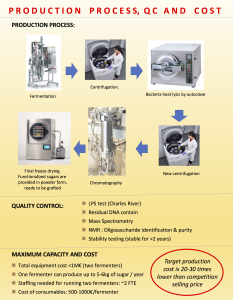
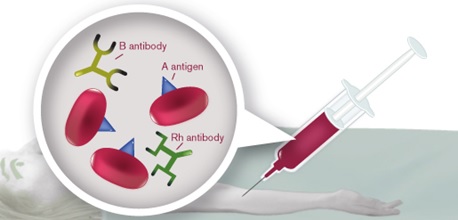
 hanks to GlycoBAR patented technology, it is now possible to produce those blood group antigen sugars in volume at a price that is low enough to envision a world where only universal plasma, injectable to any patient would exist. Economy for hospital would be huge and risk of miss match with recipient blood group would be eliminated.
hanks to GlycoBAR patented technology, it is now possible to produce those blood group antigen sugars in volume at a price that is low enough to envision a world where only universal plasma, injectable to any patient would exist. Economy for hospital would be huge and risk of miss match with recipient blood group would be eliminated.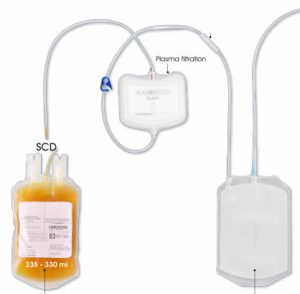 The first and most commonly used one is by using individual bags of plasma coming from individual donors (one donor = one bag). When plasma is collected, it is frozen, which is the reason why the name of this type of plasma is « FFP » for Fresh Frozen Plasma. With GlycoBAR technology, it is perfectly possible to design a filter to be included in the blood collection bag system so that antibodies are captured before the plasma is frozen.
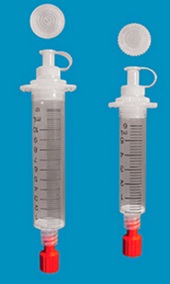
The first and most commonly used one is by using individual bags of plasma coming from individual donors (one donor = one bag). When plasma is collected, it is frozen, which is the reason why the name of this type of plasma is « FFP » for Fresh Frozen Plasma. With GlycoBAR technology, it is perfectly possible to design a filter to be included in the blood collection bag system so that antibodies are captured before the plasma is frozen. industrial treatment in a pharmaceutical environment. In such a case, there are two possibilities of removing blood group antibodies from such a plasma pool. The first one is to use a chromatographic column where GlycoBAR antigen sugars are grafted on a chromatographic resin. The plasma goes through this column, blood group antibodies are captured in the column thanks to their affinity with the antigen ligands and after each pool the column is washed with an ad hoc solution in order to be reused for the next batch. Another option is to use our disposable BAR product, which is a cellulosic support on which the antigen sugars are immobilized. When mixed with the plasma, BAR product captures the blood group antibodies and is then filtered out and thrown away.
industrial treatment in a pharmaceutical environment. In such a case, there are two possibilities of removing blood group antibodies from such a plasma pool. The first one is to use a chromatographic column where GlycoBAR antigen sugars are grafted on a chromatographic resin. The plasma goes through this column, blood group antibodies are captured in the column thanks to their affinity with the antigen ligands and after each pool the column is washed with an ad hoc solution in order to be reused for the next batch. Another option is to use our disposable BAR product, which is a cellulosic support on which the antigen sugars are immobilized. When mixed with the plasma, BAR product captures the blood group antibodies and is then filtered out and thrown away.